May 731, 7068
Authors
He L, Gomes AP†, Wang X†, Yoon SO, Lee G, Nagiec M, Cho S, Chavez A, Islam T, Yu Y, Asara JM, Kim BY, Blenis J.
Molecular Cell 2018, doi:https://doi.org/10.1016/j.molcel.2018.04.024
Abstract
Highlights
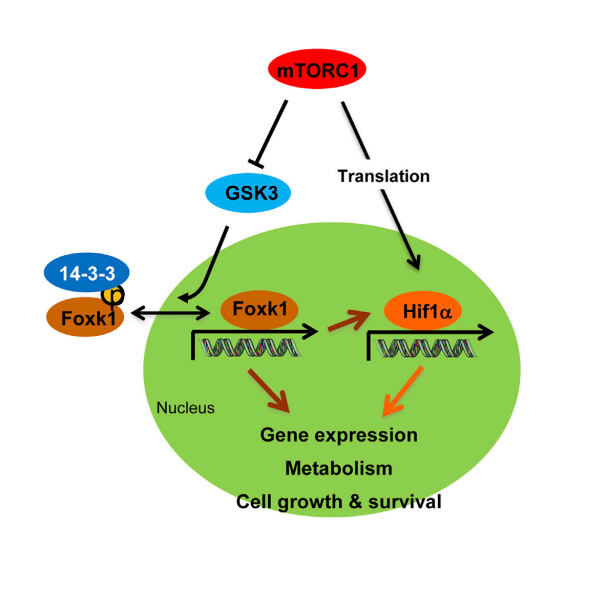
- mTORC1 suppresses GSK3-dependent Foxk1 phosphorylation
- Foxk1 phosphorylation promotes 14-3-3 binding and nuclear exclusion
- Foxk1 transcriptionally regulates Hif1α expression
- Foxk1 and Hif1α contribute to mTORC1-regulated metabolic reprogramming
Summary
The mammalian Target of Rapamycin Complex 1 (mTORC1)-signaling system plays a critical role in the maintenance of cellular homeostasis by sensing and integrating multiple extracellular and intracellular cues. Therefore, uncovering the effectors of mTORC1 signaling is pivotal to understanding its pathophysiological effects. Here we report that the transcription factor forkhead/winged helix family k1 (Foxk1) is a mediator of mTORC1-regulated gene expression.
Surprisingly, Foxk1 phosphorylation is increased upon mTORC1 suppression, which elicits a 14-3-3 interaction, a reduction of DNA binding, and nuclear exclusion. Mechanistically, this occurs by mTORC1-dependent suppression of nuclear signaling by the Foxk1 kinase, Gsk3. This pathway then regulates the expression of multiple genes associated with glycolysis and downstream anabolic pathways directly modulated by Foxk1 and/or by Foxk1-regulated expression of Hif-1α. Thus, Foxk1 mediates mTORC1-driven metabolic rewiring, and it is likely to be critical for metabolic diseases where improper mTORC1 signaling plays an important role.
Keywords
mTOR, Foxk1, Foxk2, Hif1α, GSK3, transcription, phosphorylation, metabolism